Diffusion Conclusion
Unnecessary uncertainty
For too long, there have been controversies and uncertainties about the different diffusion phenomena. This is unnecessary because the science is clear and straightforward, as described in the apps.
What follows is Charles' overview of the controversies and their conclusion, with the modeling being done with the HSPiP diffusion software.
The Charles Hansen View
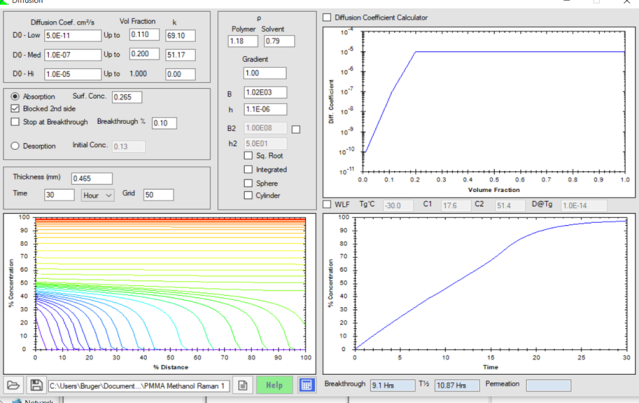 The PMMA diffusion discussion continues into 2021 with papers continuing on the topic. There is now an update to the PMMA documents below Methanol PMMA Absorption With Horizontal Concentration Gradients.docx. As the Abstract says:
The PMMA diffusion discussion continues into 2021 with papers continuing on the topic. There is now an update to the PMMA documents below Methanol PMMA Absorption With Horizontal Concentration Gradients.docx. As the Abstract says:
The absorption of methanol into PMMA follows a straight-line curve when plotted against linear time. Concentration dependent diffusion coefficients and a significant surface entry mass transfer coefficient were used in the diffusion equation to fit this curve. The results confirm a slowly rising surface concentration with concentration gradients becoming horizontal after about half of the equilibrium absorption uptake.
2020 has provided a chance for an updated overview. These documents capture the essence of the arguments below: Diffusion in Polymers 2020 Introductory text.docx and a Powerpoint version: Diffusion in Polymers 2020.pptx
The following analyses of key research dealing with absorption in polymers provide alternate, consistent interpretations to those originally proposed in the earlier studies. These analyses confirm that solutions for the diffusion equation with concentration dependent diffusion coefficients and a potentially significant surface resistance can accurately model the experiments reported.
- In short, the key experiments of Petropoulos and coworkers are based on faulty data with leakage providing a step-like absorption front. Those experiments where this is not the case are easily modeled as indicated in the apps.
- The work of Thomas and Windle is based on a tracer that diffuses much more slowly than the solvent used in the study and thus leads to the erroneous conclusion that there is a step-like, (vertical) advancing absorption concentration gradient. Modeling with the diffusion equation confirms that the concentration gradients end being horizontal rather than vertical, with getting through the surface being the controlling step. Because of concentration dependent diffusion coefficients, when the concentration of methanol in the film gets high enough, diffusion within the film is much more rapid than solvent can get through the surface. The concentration profiles necessarily become horizontal since the solvent distributes rapidly compared to the rate of entry.
- The often-cited work of Jacques, Hopfenberg and others in the same school can be modeled with the diffusion equation including a highly significant surface resistance to give a Super Case II profile, but the experimental data is misleading due to retained styrene monomer in the polystyrene films.
- The proposed mechanism by Carla and coworkers for absorption of supercritical carbon dioxide into 1 micron thick PMMA requires a diffusion coefficient that is more than one thousand times slower than that expected. Diffusion coefficients were not measured. The surface mass transfer coefficient h used to accurately model the experimental data is in good agreement with h found in other systems.
Response to the Criticism of Petropoulos and Coworkers
Feb 22, 2013
Petropoulos and Coworkers have criticized my diffusion coefficient approach for explaining “anomalous” diffusion and stated that this could not model given experiments. I attempted to respond earlier as part of a full manuscript without success. The key portions of my response regarding the key experiments are included here in the document “Response to the Criticism of Petropoulos and Coworkers”, and in part in other documents being put onto the website at the same time that are more comprehensive. It has later been determined that the diffusion coefficient for the absorbing solvent that can be calculated from the data given by Petropoulos and Coworkers is several times larger than the self-diffusion coefficient for the solvent for absorption in the stretched direction in the critical experiment. Without this leakage there would not be a step-like advancing concentration gradient. Download Response to the Criticism of Petropoulos and Coworkers.pdf.
Reinterpreting Case II Absorption in PMMA
An analysis of the Thomas and Windle data for the absorption of methanol into PMMA can be downloaded as a PDF document. This report confirms that the diffusion equation can model Case II absorption without consideration of relaxation phenomena. Step-like concentration gradients advancing at a linear rate with time have been thought to be an inherent part of Case II type absorption in polymers. These are not found in this analysis of the data for the characteristic examples of Thomas and Windle. The diffusion equation solved with an exponential concentration dependent diffusion coefficient and a significant surface condition is shown to reproduce the experimental data for weight gain at the same time demonstrating the lack of step-like concentration gradients. The surface condition appears to be particularly important in the methanol/PMMA systems studied by Thomas and Windle. Methanol “fronts” meet at the center of a free film much more quickly than the iodine tracer “fronts" that give the impression of a step-like concentration gradient. The free film is saturated with methanol at the same time as the iodine tracer gets to its center only at or near 30°C. The iodine tracer molecules diffuse much more slowly than the methanol molecules as expected from literature data, making iodine unsuitable as a tracer for this system. Download Reinterpreting Case II Absorption in Polymers.pdf.
Reexamination of Super Case II – Data of Jacques, Hopfenberg, and Others
This is an analysis of experimental data with added theoretical examples. It is shown that Super Case II absorption can be successfully modeled by the diffusion equation with exponential diffusion coefficients and a significant surface condition. There is no need to consider relaxation or related phenomena as such. Download Reexamination of Super Case II.pdf.
Reinterpreting the Experiments of Carlà et al. for the Absorption of Supercritical Carbon Dioxide into PMMA
This is an analysis of experimental data for the absorption of supercritical carbon dioxide into 1 micron thick PMMA films applied to quartz microbalances. It is shown that a significant surface condition completely controls the absorption with flat concentration gradients in the film from the very start. Download Interpreting Absorption of Supercritical Carbon dioxide in PMMA.pdf.